Ionic or Covalent Electron Dot Structure Review Answers
1.2: Lewis Theory of Bonding
- Page ID
- 83474
Chemic bonding is at the centre of understanding chemistry. The Lewis Theory of Bonding essentially combined observations at the fourth dimension about chemic bonding together. Not only was it essential in agreement how elements bonded, it provided a visual representation for them. These Lewis dot structures are a simplistic way of representing the electrons in molecules. This theory also helped to ascertain formal charge and resonance. Understanding of electronegativity and electron affinity will be useful before understanding Lewis Theory of Bonding.
Introduction
The Lewis Theory used observations from chemists and physicists to form a theory nigh chemic bonding. This work was substantially a compilation of the cognition at the fourth dimension. It revolved around the importance of valence electrons in chemic bonding. These are the electrons that are in the outermost shell. For example Na may have 11 electrons, but simply one is a valence electron, the one in 3sone. Meanwhile P has 15 electrons, but has five valence electrons, 3s2 and 3p2. The bonding of an element is based on how they fill their octets i.eastward. achieve a noble gas electron configurations.
Lewis went on to explain how certain elements such equally Boron did not necessarily follow these same rules. He explicitly divers two ways electrons were used to form bonds. These were ionic (consummate transfer of electron) and covalent (sharing of electrons) bonding. He added to his covalent bonding theory that atoms can have a double or triple bonds with other atoms when electrons are shared. A single bond consists of ii electrons sharing between two atoms, double bail consists of iv electrons sharing betwixt ii atoms, and triple bond consists of six electrons sharing between two atoms. In both cases Lewis dot structures were used to visualize the the bonding of the atoms in example of electron sharing or electron transfer.
Basic Lewis Dot Structures
There are two basic types of bonding that grade the footing of the Lewis theory and it is important to understood before ane can begin to draw Lewis dot structures.
Ionic Bonding
In ionic bonding one element is much more electronegative then another element. In other words, the electronegative atom wants an electron much more the other element; thus, the element with a positive charge loses electron and the element with the negative charge gains an electron. These two elements so get attracted to each other because of Coulomb'due south Law which states that at that place is electrostatic interaction between particles that are electrically charged, in this case the electrons describes the charge of the atom. The atom that loses electrons is electron deficient; thus, the atomic charge volition be positive. The atom that gains electrons has excess of electrons; thus, the diminutive charge will be negative. This allure between the elements brings them together to course an ionic bail. An instance of an ionic compound is shown beneath.
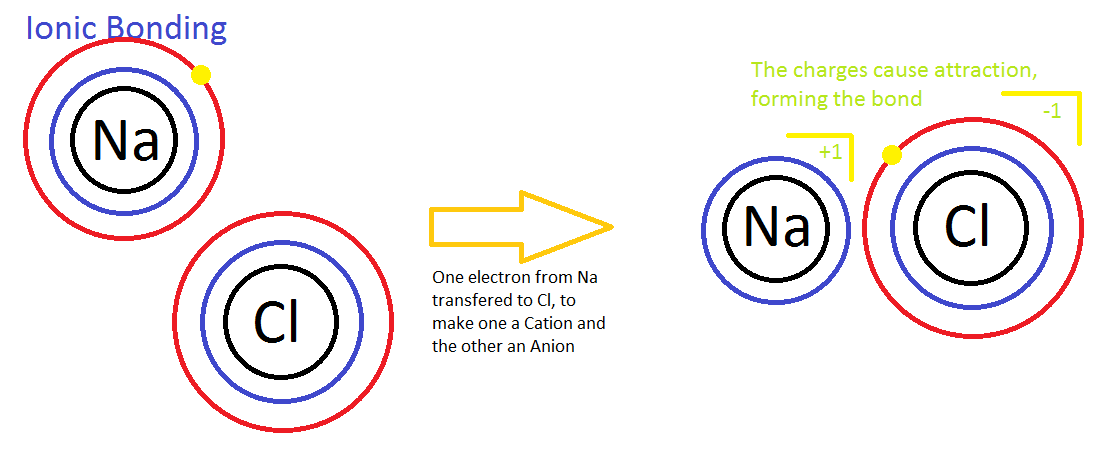
Covalent Bonding
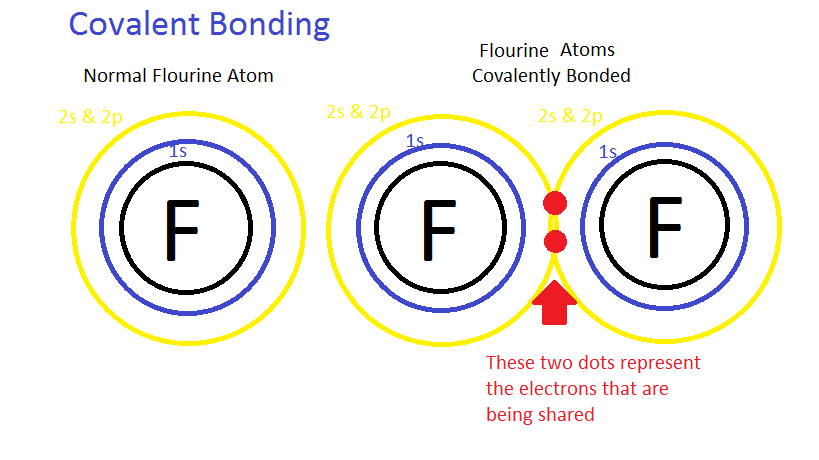
In covalent bonding the elements share electrons equally, i.e. both the atoms have an equal share of electrons in the bond. This sharing of electrons creates a bail that is known every bit a covalent bail. It is thought that a covalent bond is equal, but in nature, the bond is never equal. The electronegativity differs betwixt all the elements and the more electronegative the atom, it has a higher tendency to proceeds electrons; however, because the bond is formed past sharing electrons, the atom cannot "possess" an electron. So, the only way for the more electronegative atom to take the "reward" of an electron is by unequal sharing of electrons. The compounds that share electrons unequally are called polar compounds and the procedure is chosen polarity. An example of a polar compound is water (H2O). An example of a generalized covalent chemical compound is shown beneath.
How to Draw Lewis Structure: To begin any Lewis dot structure, begin by counting how many valence electrons are in all the elements.
CHiv CO2
1 C: 1 * [4 Valence Electrons] (2s22p2) i C: 1 * [4 Valence Electrons] (2s22p2)
4 H: 4 * [1 Valence Electron] (1s1) ii O: 2 * [six Valence Electrons] (2s22p4)
4+4 = eight Valence Electrons 4 + 12 = 16 Valence Electrons
Adjacent, arrange the elements in a bones outline, without the electrons or bonds. This is sometimes known equally a skeleton structure. In general the least electronegative element goes in the middle, and hydrogen is almost e'er on the outside.
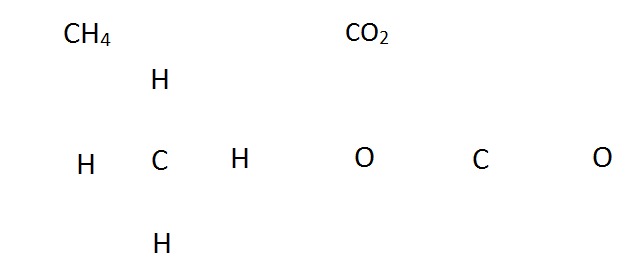
Finally, connect the elements, remembering that hydrogen only needs 2 electrons and other elements should have viii. A good strategy is to first connect them with single bonds and fill in the rest with electrons. If in that location are not enough electrons add in double/triple bonds when necessary.
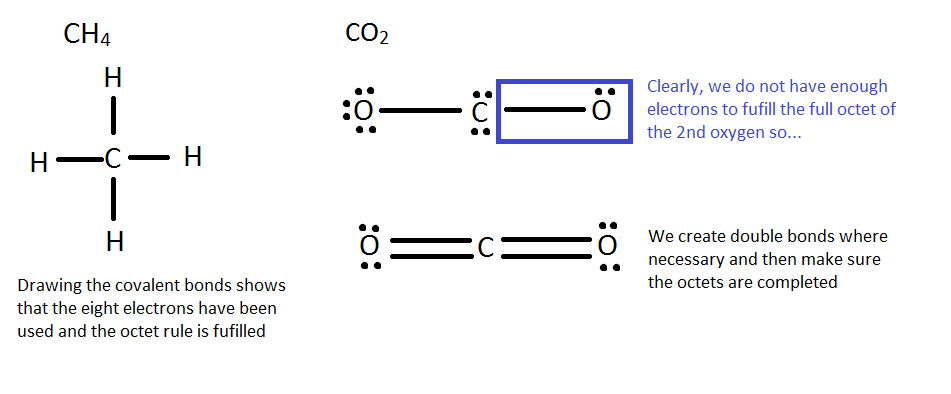
The essential goal is to always ensure that each element has a full octet and the molecule has all the valence electrons it should have from its constituents.
Formal Charges
In an atomic bond, either ionic or covalent, atoms proceeds or lose electrons. The formal charge is the difference between the original number of valence electrons and the actual number of electrons used in filling the octet. The actual number of electrons is the addition of the solitary pair electrons and one electron from each bond. Just one electron is counted in a bail because there are ii electrons existence shared in the bond merely only one is being possessed by each atom.
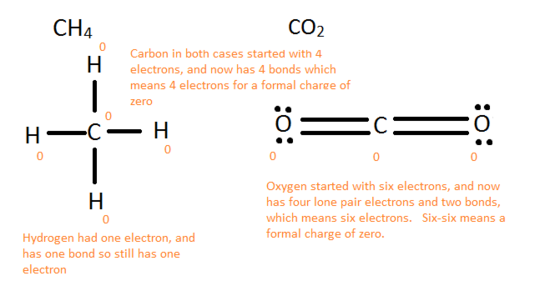
The following rules are needed to see if a item Lewis structure can be possibly formed in nature.
- Formal charge of 0 --> Actually good, possible in nature
- Formal accuse of -1 or i --> Okay, possible in nature
- Formal accuse < -2 or > 2 --> Not practiced, incommunicable in nature
To bank check if the formal charge for a detail compound is correct, one tin check if the full of all formal charges of the atoms in the compound equal to the accuse of that compound. Ane instance tin be seen in the 2nd effigy of category "Resonace" beneath.
Resonance
Draw a Lewis dot construction for [SCN]-. Is it i of the ones beneath?
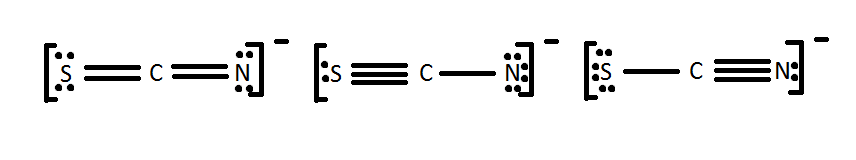
All of these molecules look different, but they fulfill the simple rules of Lewis dot structures. They all fulfill the octet dominion and apply 16 electrons (fifteen valence electrons +1 from the -). This demonstrates that in certain cases, molecules can take the same elements but information technology tin can be arranged differently by the Lewis dot theory. The different Lewis structures are called resonance structures. It is of import to recognize resonance structures only one also has to consider the natural chance of each i occurring in nature. One method is to look at the formal charges equally shown below.
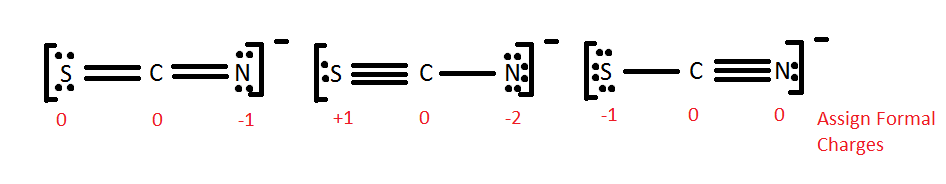
One can immediately see that it is extremely unlikely for the middle one to occur because of the formal charge of -2 to 1 of the atom i.eastward. Nitrogen atom. The formal accuse means that nitrogen will accept more than electrons and so sulfur volition become positive. By comparison the ii formal charges on the terminal atoms, one can encounter that, in either instance, sulfur has a charge of 0 or -1. This is same for Nitrogen. At present understanding the concept of electronegativity and electron affinity, which atom is more likely to have a negative charge? To answer that question we must look at electron affinities. Nitrogen has a college electron analogousness, and thus is more likely to have a negative accuse. The first structure is well-nigh common, whereas the middle structure is least probable structure for SCN-.
Questions
- The Lewis Theory focuses on ________ electrons and the _____ rule for determining bonding betwixt elements.
- Write the Lewis Dot Structure for \(MgCl_2\).
- Write the Lewis Dot Construction for \(C_2H_6\).
- Write the Lewis Dot Structure for \(CO_3^{two-}\).
- Write the formal charge for the iii elements above.
Answers
1. The Lewis Theory focuses on valence electrons and the octet rule for determining bonding between elements.
2.
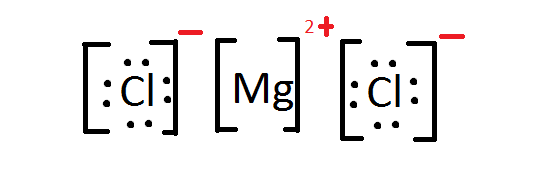
3.
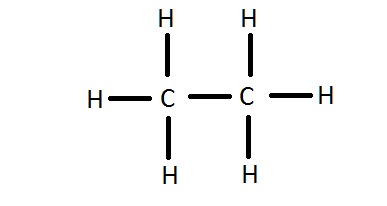
4.
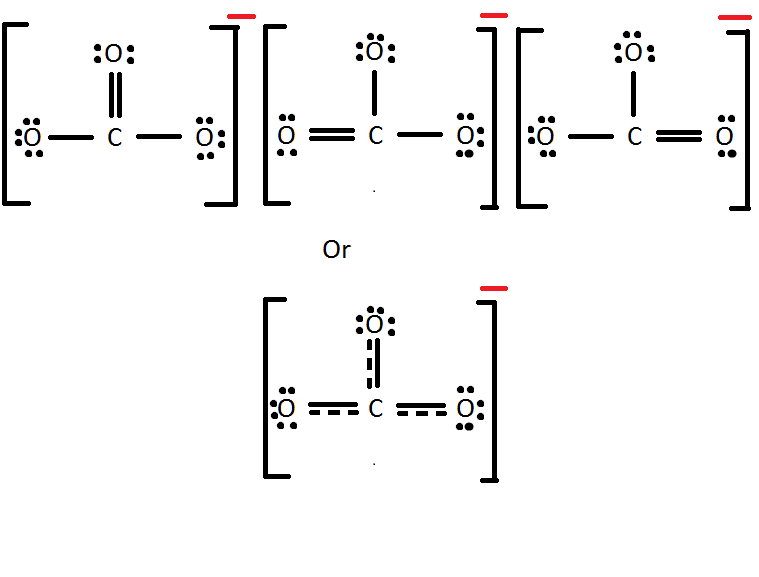
v. Mg2 + has a formal charge of two+ while Cl- has a formal charge of 1-. Carbon has a formal charge of zero and and then does hydrogen. Carbon has a formal accuse of 0 and oxygen a formal charge of -4/3.
References:
- Lewis, Gilbert Newton. Valence and the Structure of Atoms and Molecules,. New York: Chemical Catalog, 1923. Impress.
- Pauling, Linus. The Nature of the Chemic Bond: and the Structure of Molecules and Crystals : Introduction to Mod Structural Chemistry. Ithaca, NY: Cornell Upwards, 1960. Print.
- Petrucci, Ralph H. General Chemistry: Principles and Modern Applications. Toronto, Ont.: Pearson Canada, 2011. Print.
Contributors
- Nilpa Shah (UCD)
{{ Template.PageBottom() }
chandlercoarestligh.blogspot.com
Source: https://chem.libretexts.org/Courses/Douglas_College/DC:_Chem_2330_%28O%27Connor%29/1:_Introduction_and_Review/1.2:_Lewis_Theory_of_Bonding
0 Response to "Ionic or Covalent Electron Dot Structure Review Answers"
Post a Comment